“I think we will be able to deliver personalized cancer vaccines against multiple different tumor types to people around the world,” Dr. Paul Burton
The mRNA technology on which vaccines against the coronavirus were based appears to pave the way for the creation of vaccines and treatments for cancers, cardiovascular disease, autoimmune and other conditions.
The scientific community has made real leaps and bounds in this field in recent years, and information about various programs and research “running” was known. No one, however, had taken responsibility to speak with specific figures and with specific dates. The news, which concerns billions of people now and in the future, was given by its chief medical officer Modern, Dr. Paul Burton, who spoke of great things to come. Dr. Burton he said that his company will be able to present vaccines for cancers, cardiovascular and autoimmune within the next five years and definitely by 2030 they will be available to the general public.
Dr. Paul Burton
The mRNA vaccine for the coronavirus contributed to this, which showed the way and opened the door to 15 and 20 year studies, to be revealed within a period of 12 and 18 months.
“We will have this vaccine and it will be extremely effective and save many hundreds of thousands, if not millions, of lives. I think we will be able to offer personalized cancer vaccines against multiple different types of tumors to people around the world,” said Dr. Burton to Guardian.
He also said multiple respiratory infections could be covered with a single injection – allowing vulnerable people to be protected from Covid, flu and respiratory syncytial virus (RSV) – while mRNA treatments could be available for rare diseases for for which there are currently no drugs. mRNA-based therapies work by teaching cells how to make a protein that activates the body’s immune response to disease.
“I think we’re going to have mRNA-based treatments for rare diseases that were previously untreatable, and I think 10 years from now, we’re going to be approaching a world where you can actually identify the genetic cause of a disease and, with relative simplicity, to address it, using mRNA-based technology.”
But scientists warn that the accelerating progress, which has increased “by orders of magnitude” over the past three years, will be lost if the high level of investment created by the pandemic is not maintained.
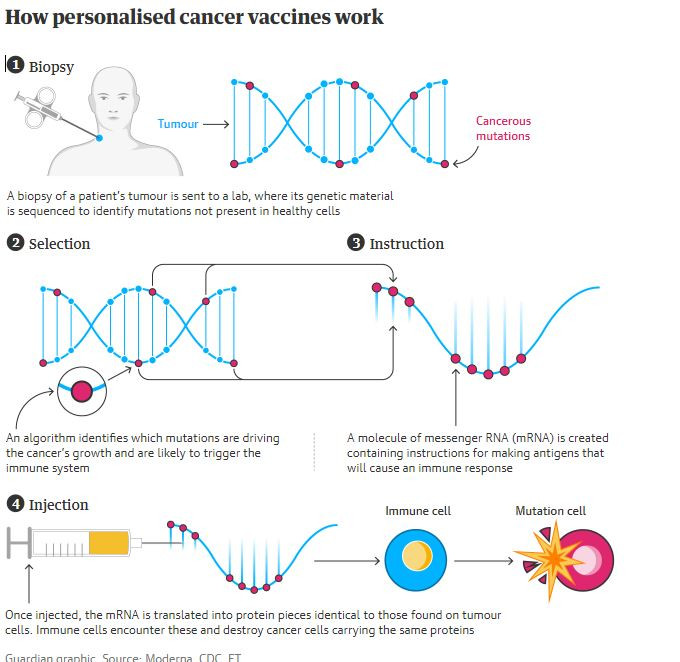
The mRNA molecule instructs cells to make proteins. By injecting a synthetic form, the cells can pump out the proteins we want our immune system to attack. An mRNA-based cancer vaccine would alert the immune system to a cancer already developing in a patient’s body so it can attack and destroy it, without destroying healthy cells.
This involves identifying protein fragments on the surface of cancer cells that are not present on healthy cells – and which are more likely to trigger an immune response – and then creating pieces of mRNA that will instruct the body on how to make them.
First, doctors take a biopsy of a patient’s tumor and send it to a lab, where its genetic material is analyzed to find mutations that aren’t present in healthy cells.
A machine learning algorithm then determines which of these mutations are responsible for the development of cancer. Over time, it also learns which parts of the abnormal proteins these mutations encode are most likely to trigger an immune response. Antigens are then made using mRNA technology and packaged into a personalized vaccine.
Dr. Burton said: “I think what we’ve learned in the last few months is that if you ever thought that mRNA was just for infectious diseases or just for Covid, the evidence now shows that’s not the case. It can be applied to all kinds of diseases. In cancer, infectious diseases, cardiovascular diseases, autoimmune diseases, rare disease. We have studies in all of these areas and they have all shown tremendous promise.”
In January, Moderna announced results from a late-stage trial of its experimental RSV mRNA vaccine, suggesting it was 83.7 percent effective in preventing at least two symptoms, such as cough and fever, in adults age 60 and older. Based on these data, the US Food and Drug Administration (FDA) has granted breakthrough vaccine therapy designation, meaning its regulatory review will be expedited.
In February, the FDA granted the same designation to Moderna’s personalized cancer vaccine, based on recent results in patients with the skin cancer melanoma.
“I think it was an order of magnitude that the pandemic accelerated this technology. It’s also allowed us to scale up production, so we’re extremely good at producing large quantities of vaccines very quickly,” Moderna’s chief medical officer emphasized.
Source :Skai
I have worked in the news industry for over 10 years. I have a vast amount of experience in covering health news. I am also an author at News Bulletin 247. I am highly experienced and knowledgeable in this field. I am a hard worker and always deliver quality work. I am a reliable source of information and always provide accurate information.