The findings of the study could contribute to the discovery of drugs for Alzheimer’s disease and other neurodegenerative diseases
‘Light’ in mysterious areas of pathological amyloid fibrins, proteins responsible for the Alzheimer’sfor the first time she throws an international research team, opening ways to discover new drugs for the disease. Head of research, published in the magazine ‘Science Advances’is the Dr. Zacharias Faidon Brotzakis From EKEVE Fleming.
Alzheimer’s disease, as well as several other neurodegenerative diseases, is characterized by the formation of amyloid fibrins. “Characteristic of Alzheimer’s disease is the formation of insoluble amyloid fibrins of AB42 protein, which in turn are stacked in large deposits, known as amyloid plates, which are mainly formed in the brain cortex and in the hippopotamus area. Stress, neuronal destruction, as well as underfunding of synapses, affecting memory and learning. At an atomic level, smaller oligomers and amyloid fibrils also constitute toxicity agents by interacting with cell membranes, disrupting cellular homeostasis, affecting synaptic function and activating neurophariosis. “Mr. Brotzakis, a postdoctoral researcher at EKEVE Fleming, in the workshop of the Director of the Institute of Innovation, Dr. Shrata.
These fibrils include a structurally stable β-fold amyloid nucleus surrounded by extremely dynamic inherently disturbed areas, known as “Fuzzy Coat”. Although it is believed that these areas affect Alzheimer’s pathology, their dynamic nature has made their study difficult and scientists have so far knew the structure of their structured section.
“However, only the characterization of the structures adopted by all amyloid fibers can reveal to us the physicochemical properties associated with their solubility, a decisive factor that contributes to their toxicity and pathology,” explains Brotzakis. He adds, “with this research we characteristically characterize the fiber as a whole for the first time and take a step towards decoding the secrets of the disease and its treatment.”
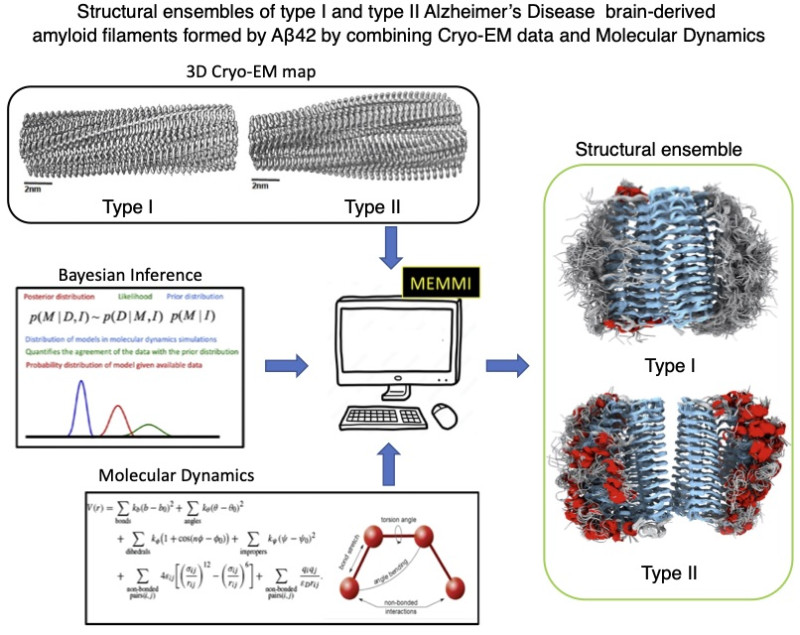
The research team, which includes the Professor of Biophysics Michele Vendruscolo and Dr. Maria Milanesi from the Department of Chemistry at the University of Cambridge, used an innovative methodology called “Metadynamic Electron Microscopy Metainference” (Memmi), which was invented by Vendroussolo and Brotzakis. This method combines Cryo-EM Cryo-EM data, a cutting-edge technology with the help of which scientists can study the biomolecules with very precision, as well as computing molecular dynamics, to characterize the dynamics of AB42 , occurring in brains of patients with Alzheimer’s.
By this method The group analyzed two types of amyloid fibrils AB42, which are associated with the familial (or hereditary) form of Alzheimer’swhich represents less than 5% of Alzheimer’s cases, and the sporadic form, which is found in 90-95% of patients and is associated with a complex interaction of genetics, environmental and lifestyle factors. The study revealed that family -related fibrils are less soluble than sporadic fibrils. In addition, Fuzzy Coat appeared to contribute to increasing the solubility of both types of fibrins.
Commenting on the importance of the findings, Professor Michel Venteruskolo points out that “in the study we have managed to determine the structure of certain mysterious areas of amyloid fibrin found in the brains of patients with Alzheimer’s. We have shown that the most aggressive form of Alzheimer’s disease, which affects individuals from the age of 30-50, is associated with a particularly insoluble type of amyloid deposits. Since amyloid deposits are the goal of currently approved drugs for Alzheimer’s disease, our results indicate that even more effective drugs could develop if they aim to eliminate the most insoluble amyloid forms, so new opportunities are offered.
The study provides the basis for further research on the role of Fuzzy Coat in Alzheimer’s pathology. It also highlights the potential of the Memmi method in order to characterize the dynamics of pathological biomines.
The findings of the study could contribute, as Mr Brotzakis explains to RES-EIA, to the discovery of drugs for Alzheimer’s disease and other neurodegenerative diseases, which are “structure-based”, that is, planned on the basis of its structure. Their biological goal and thus be able to intervene in their further formation. He even uses a parallel to better understand the importance of this discovery in finding new drugs. “The classic mechanism of pharmaceutical discovery recommends finding the key that fits a lock, target protein. In proteins, such as those we study which develop inherent dynamics, you must actually find a key, a chemical molecule, which unlocks different locks, that is, different structures adopted by the target protein due to its inherent dynamics. “
The next goal of the researchers is to find such molecules possible that they will be the key to halt the formation of amyloid fibers and thus, the development of the disease and which will be further tested in experimental preclinical studies. They also intend to extend their study to other proteins associated with neurodegenerative diseases, such as Parkinson’s and ALS.
Source :Skai
I have worked in the news industry for over 10 years. I have a vast amount of experience in covering health news. I am also an author at News Bulletin 247. I am highly experienced and knowledgeable in this field. I am a hard worker and always deliver quality work. I am a reliable source of information and always provide accurate information.