Of Olympian Papadimitriou
Pharmaceutical research is evolving at a very intensive rhythm worldwide, and pharmaceutical innovation literally changes patients’ lives, increasing life expectancy and giving improved quality of life, which can mean less pain, a normal daily life, returning to work and much more.
As pharmaceutical companies we operate in Greece we have as our main concern Make this innovation, as soon as possible, available to the Greek patient.
The environment, however, that has been formed after the financial crisis and the Memorandums, makes our mission more and more difficult. The timeless underfunding of the public budget for the drug, it stands unparalleled obstacles to access to Greek patients to new innovative drugs. Its consequence are dozens of new innovative drugs in Europe, significantly improving the daily life and quality of life of thousands of patients, absent from our country, with Greek citizens hoping that at some point in the near future they may have the same right to access.
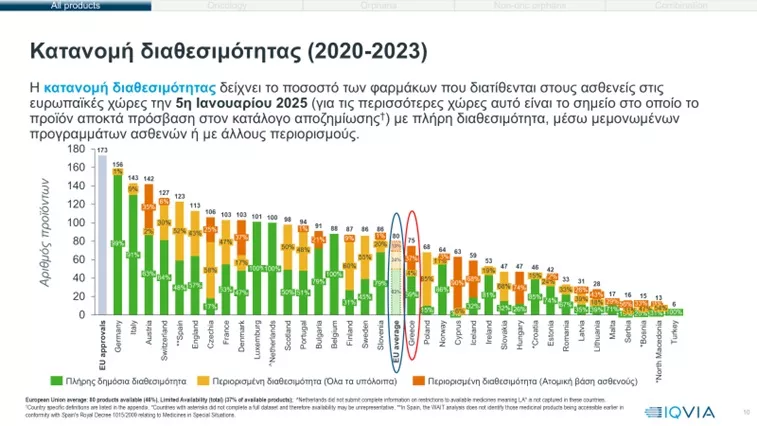
The Study of Patient Wait Indicator, which has been entrusted with the European Federation of Pharmaceuticals and Associations (EFPIAs) for 20 years, is demonstrating persistent and significant differences and inequalities in patients’ access to new drugs between countries across the European Union. Especially in our country, fewer new innovative medicines are coming and even delaying almost two (2) years after their approval by the European Medicines Agency (EMA). Of the 173 new, innovative drugs that were approved by the European Medicines Agency (EMA) from 2020 – 2023, Only 75 are circulating in our countryof which 59% are universally accessible and the rest with a limited mood (That is, they come to our country through SPP or IFET or private).
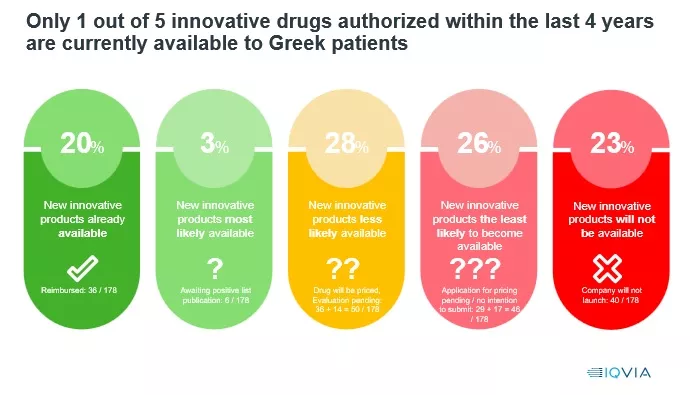
Trying to capture the Greek reality for the first year, for the second consecutive year, SFEE commissioned IQVIA to design a study on the availability of new drugs in Greece between 2021-2024, which showed that of 178 new innovative drugs that took approval of 20-2021 Only 36 (20%) are currently (31/03/2025) available in the Greek market (finding similar to last year), That is, only 1 in 5 innovative drugs of the last four years is currently available to Greeks patients. In other words, 4 out of 5 new, innovative drugs do not come to our country at first and may come later.
The dignified business climate formed by the excessive compulsory returns has resulted in many new innovative medicines not available to Greek patients in the near future and hope for better treatment is transferred to the distant future, at least for many. International pharmaceutical companies that basically drive pharmaceutical research and develop pharmaceutical innovation, unable to provide many new drugs in our country under existing extreme over -taxation conditionsdue to mandatory returns.
Government commitments promise a better future for pharmaceutical businesses, but the numbers of the present as well as the huge delays in settlement of accounts between state and companies do not allow optimism, intensify uncertainty and abolish any predictability.
SFEE, through the 63 companies – 17 Greek, 46 international, productive, and innovative, represents over 90% of the pharmaceutical market in Greece. The pharmaceutical industry is a key pillar of the Greek economy as It contributes to 6.2 billion euros to the national economy (3.4% GDP) and, it supports more than 108,000 jobs.
And while the contribution of pharmaceutical innovation to the health system and well -being of citizens is unfortunately increasing, political recognition and integration of decisions is lacking – for more than 15 years. It’s time to change that.
Our society, the NHS, needs a truly innovative environment that rewards research, evolution, extroversion and above all the value that the pharmaceutical industry returns to the patient and society.
Inequality in access to Greek patients to new innovative treatments should be stopped at some point and this can only be done with substantial funding for the drug and some brave reform policies that are constantly delayed.
It is therefore necessary to reassess the investment in the drug, and to view it as a tool to stop other expense health system (hospitalizations, surgery, chronic complications, etc.). And After installing the perception that the funding of the drug is an investment, the yields of this investment should be significantly increased. The tools are mainly available through the digital reform of health, the complete application of therapeutic protocols, the digital patient dossier, the integration of laboratory tests into the electronic prescription and its expansion into hospitals, the completion of patient registries, the increase in and compensation for new treatments and of course the exemplary punishment of all kinds of bad practices where they are found.
SFEE and its pharmaceutical companies are trying to find the golden intersection between the increasing needs for pharmaceutical care and the limited resources to finance these needs with a common vision: a healthier, more durable, more fair. The challenges are there for all European health systems. Consultations with the Ministry of Health on the signing of a cooperative agreement (MOU), which will enhance transparency, define co -responsibility and have clear and realistic commitments for each side will continue to be accompanied by substance.
* Olympios Papadimitriou is President of SFEE
Source :Skai
I have worked in the news industry for over 10 years. I have a vast amount of experience in covering health news. I am also an author at News Bulletin 247. I am highly experienced and knowledgeable in this field. I am a hard worker and always deliver quality work. I am a reliable source of information and always provide accurate information.