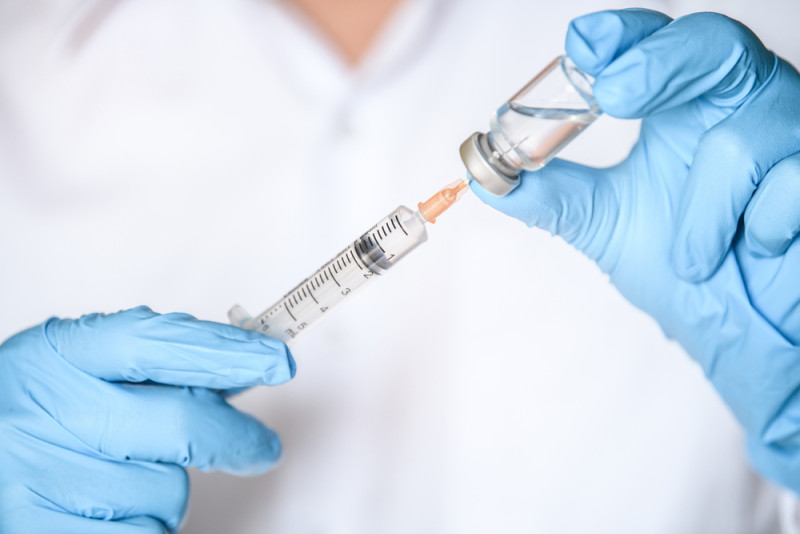
Each injection will be completely personalized for the patient
Dr. Thomas Wagner, founder of the biotech company Orbis Health Solutions and a cancer researcher, has made it his life’s mission to find a way to cure cancer without the terrible side effects that, for some, can make it worse than the cancer itself or even lead to premature death.
“The tragedy of cancer is also about the fear of treatment,” he told ABC News.
Many traditional cancer treatments, such as chemotherapy, work by killing cancer cells, but they also kill non-cancerous cells throughout the body. This can cause a number of side effects such as hair loss, nausea, vomiting, or it can suppress a person’s immune system, putting them at risk.
After seeing cancer patients suffer from debilitating side effects of their treatment, the researcher set out to develop a cancer treatment that harnesses the power of a person’s immune system instead of neutralizing it. This treatment was developed as a vaccine that has now been studied for decades and each injection is completely individualized for each patient.
What we know so far about this cancer vaccine
The TLPO cancer vaccine has been tested in hundreds of patients with advanced forms of melanoma in Phase 2 clinical trials.
The most recent data presented at an academic conference showed that almost 95% of people who received the vaccine alone were still alive three years after starting treatment, and 64% were still disease-free (if someone lived five years after the cancer is treated, then he is disease free).
Among the more advanced forms of melanoma, disease-free survival after three years for people with stage III disease was 60% in the group that received the vaccine alone, compared with about 39% in the placebo group. Disease-free survival for people with stage IV disease was about 68% in the vaccine-only group and zero in the placebo group.
The most common side effects were redness or pain at the injection site, fever and tiredness after the injection – similar to other vaccines that stimulate an immune response.
Dr. Vernon Sondak, a cutaneous oncologist at Moffit Cancer Center, who was not involved in the clinical trial, told ABC News that these results are promising, but notes that phase 2 clinical trials are inconclusive. A larger Phase 3 clinical trial should ultimately validate whether this cancer vaccine will truly be a game-changer in the field.
“We’ve seen time and time again, promising Phase 2 data that didn’t turn out to be so promising in Phase 3,” warns Sondak.
Based on these data and other studies, the Food and Drug Administration (FDA) gave Wagner’s vaccine the green light to begin a phase 3 clinical trial. It will be a three-year project, with the goal of enrolling 500 people, and is planned to will begin sometime this year, said Riley Polk, president of Orbis Health Solutions.
Polk said he has been personally affected by the success of this vaccine since his father underwent multiple lung operations for cancer a decade ago but had no other treatment options. His father chose to try Wagner’s cancer vaccine and lived another 10 years before dying of something unrelated to cancer. “You can tell me a lot of things, but you can’t tell me the vaccine doesn’t work,” Polk said.
Economic obstacles held back progress
Polk said the planned phase 3 clinical trial is a $100 million project. These funds, according to him, are crumbs for pharmaceutical companies and those backed by venture capitalists.
For smaller, private companies, securing that level of financial resources is a challenge that Polk says limits their ability to fund more clinical trials that could expand potential indications for Wagner’s cancer vaccine.
To circumvent some of this challenge and give this treatment to more people that might yield more results, Wagner and his team have just begun what’s called a “basket” trial, a type of clinical trial approved by the FDA and allows the same vaccine—which has shown success in clinical trials for melanoma—to be tested on anyone with a solid tumor who meets certain inclusion criteria. People in this trial must have a low or minimal tumor burden, so most will have already received some kind of treatment before receiving this vaccine, Wagner said.
The first person to receive this vaccine, as part of this “basket” trial, was Catie King, a resident of Asheville, North Carolina, who was diagnosed with ovarian cancer six years ago. King said she felt great after the first round of treatment, experiencing only some redness at the injection site, but no other side effects. For King, who farms with her husband, the lack of side effects makes a real difference in her life as it doesn’t affect her daily life at all. “With this vaccine, there were no hard days,” she said, which was not the case when she was undergoing chemotherapy for her cancer.
Polk said he hopes the data from the “basket” trial will attract the attention of larger drug companies. Through this kind of collaboration and with other funding mechanisms they could eventually do more extensive testing through the FDA.
Source :Skai
I have worked in the news industry for over 10 years. I have a vast amount of experience in covering health news. I am also an author at News Bulletin 247. I am highly experienced and knowledgeable in this field. I am a hard worker and always deliver quality work. I am a reliable source of information and always provide accurate information.