The recall comes because after checks it emerged that due to the use of the specific products, there may be a risk for the development of the embryos.
The EOF announced the recall of batches of three products, citing risks for the development of fetuses in pregnant women.
The recall comes because after checks it emerged that due to the use of the specific products, there may be a risk for the development of the embryos.
In particular, the National Medicines Agency announced the recall of batches of the medical devices in the table below after informing the manufacturer FUJIFILM Irvine Scientific Inc as toxicity of the oil of the crop was detected which may cause damage to the development of the embryo and insufficient blastocyst development or non-transferable blastocyst resulting in the impossibility of carrying out the procedure with the weakened fetus.
The recall concerns the following products and batches
The decision is a precautionary measure to reinforce the voluntary recall undertaken by the manufacturing company.
Customers of the products in question in Greece must implement the recall and the actions proposed by the company immediately. The documents of the revocation must be kept for a period of at least five (5) years and brought to the attention of the EOF, if requested.
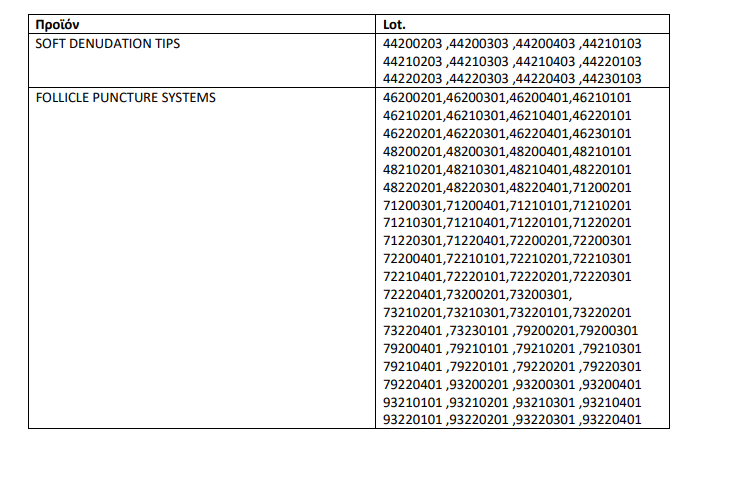
Recall of additional lots
At the same time, the EOF decided to recall the batches of the following table of MDD Class IIa medical technology products, SOFT DENUDATION TIPS + FOLLICLE PUNCTURE SYSTEMS due to the fact that, during the risk assessment, the safety of the products cannot be demonstrably confirmed, especially as regards concerns embryotoxicity.
This decision is issued in order to reinforce the voluntary recall undertaken by the manufacturing company. Recipients must follow the instructions provided by the manufacturer regarding the withdrawal and fate of the products in question. The documents of the revocation must be kept for a period of at least five (5) years and brought to the attention of the EOF, if requested.
Source :Skai
I have worked in the news industry for over 10 years. I have a vast amount of experience in covering health news. I am also an author at News Bulletin 247. I am highly experienced and knowledgeable in this field. I am a hard worker and always deliver quality work. I am a reliable source of information and always provide accurate information.